Dow Corning Silicone Breast Implant
BAPRAS/140
1972
What Is This Device?
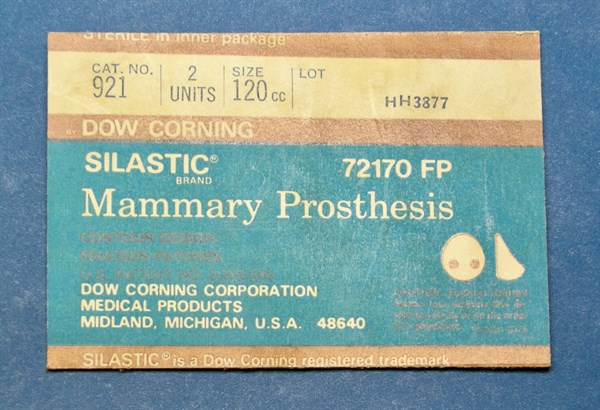
Anatomically shaped, Silicone filled “Silastic” breast augmentation prosthesis of 120cc volume.
Measuring 135mm length, 105mm, wide and projecting (height) a maximum of 40mm. The device has Dacron fabric patches attached to the posterior (chest-facing) surface.
What Is It Used For?
This implant was used for cosmetic breast augmentation. Placed through an incision under each breast it was initially designed to be located below the breast glandular tissue and above the chest (pectoral) muscles. This particular device was used in the early 1970's for cosmetic breast augmentation, by Brian Morgan. It was explanted again in 1987 for an unknown reason.
Many of these early devices ruptured, or were encased within tough scar capsule. This changed their shape and/or made them uncomfortable enough to prompt their removal. This remains a complication of the surgery today. This device is intact, but mildly “greasy” on the surface due to slow” gel bleed” through the Silastic envelope. This was also an issue with early breast implants whose envelopes were more porous than today.
Significance To Plastic Surgery
Dow Corning implants very similar to this were the first practical breast implant devices available to plastic surgeons. Dow Corning of Michigan, USA, registered the trademark “Silastic” in 1948 as a flexible silicone sheet (elastomer). Together with more liquid polymeric silicones (Dimethyl Siloxane) it had a range of industrial applications before being used in various medical and pharmaceutical devices.
Dow Corning produced the first practical silicone membrane enveloped, silicone-filled, breast implant to the specification of plastic surgeons Thomas Cronin and Frank Gerow in 1962. They first used one of their implants in a patient in Houston the same year. Their device was very similar to the example shown here, being anatomically-shaped and having a textured patch of Dacron on the rear. This was designed to stop the implant moving, and particularly rotating upside down. These patches were later abandoned due to concerns that they were increasing the formation of tough and distorting scar capsule around the implants.